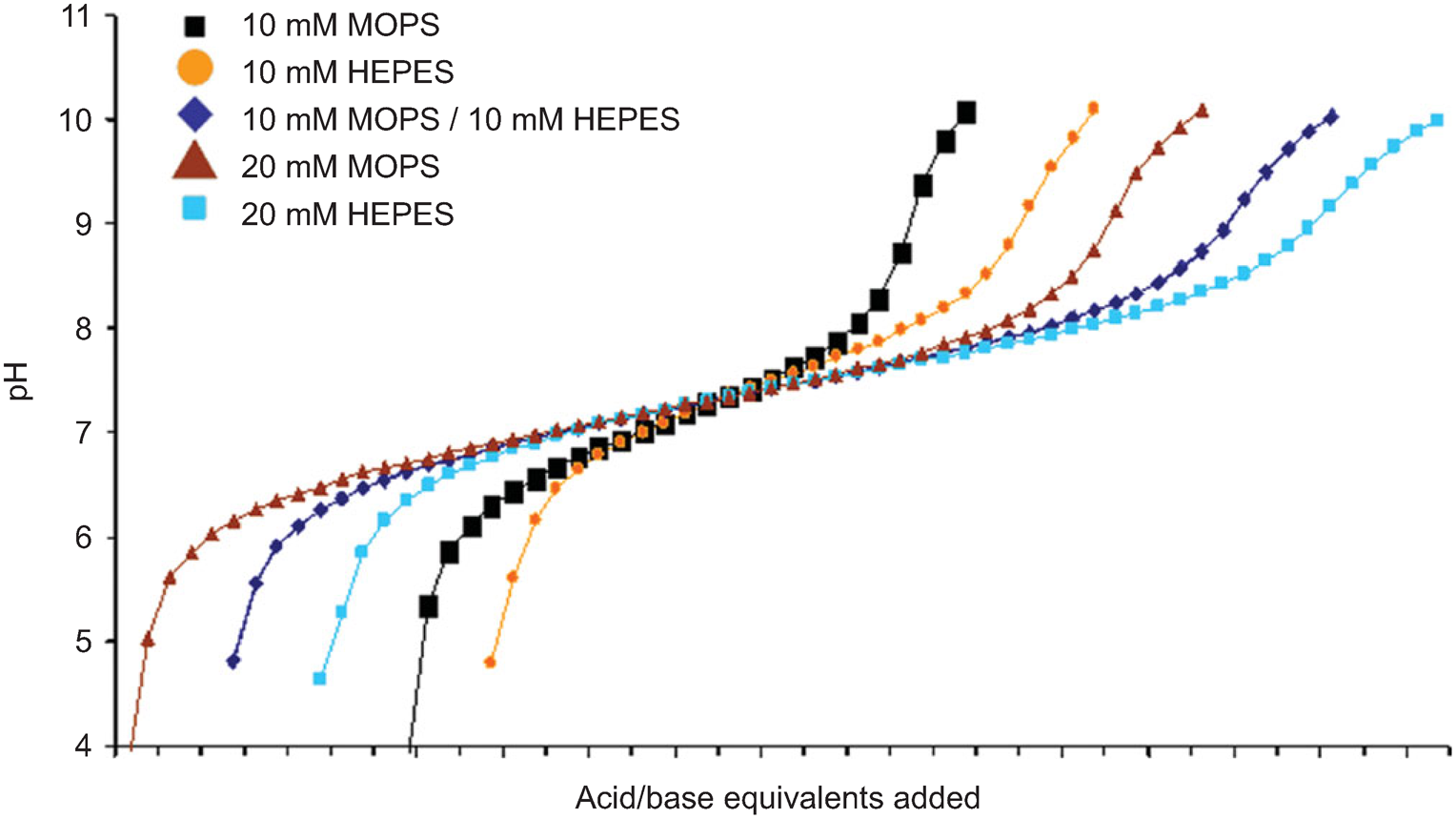
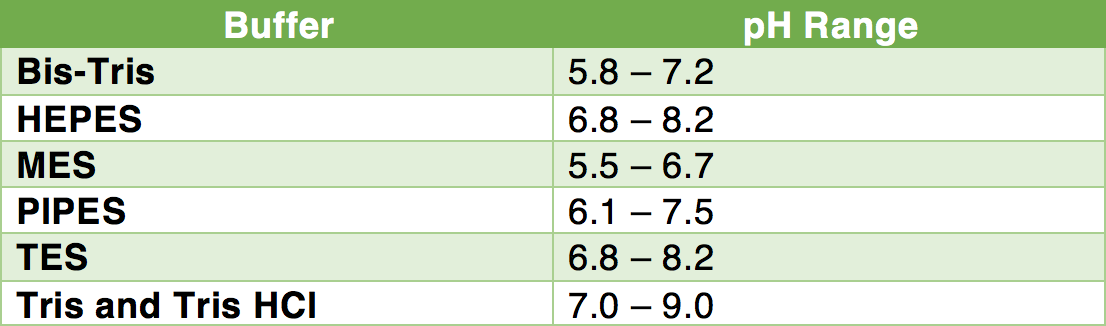
Buffer stability. (a) SenALiB was diluted in different buffers with... | Download Scientific Diagram

Effect of Tris, MOPS, and phosphate buffers on the hydrolysis of polyethylene terephthalate films by polyester hydrolases - Schmidt - 2016 - FEBS Open Bio - Wiley Online Library

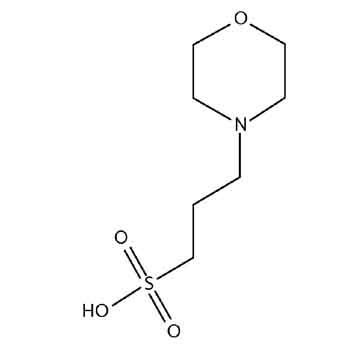
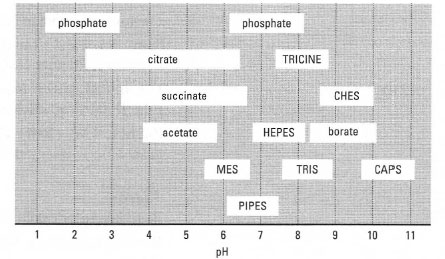
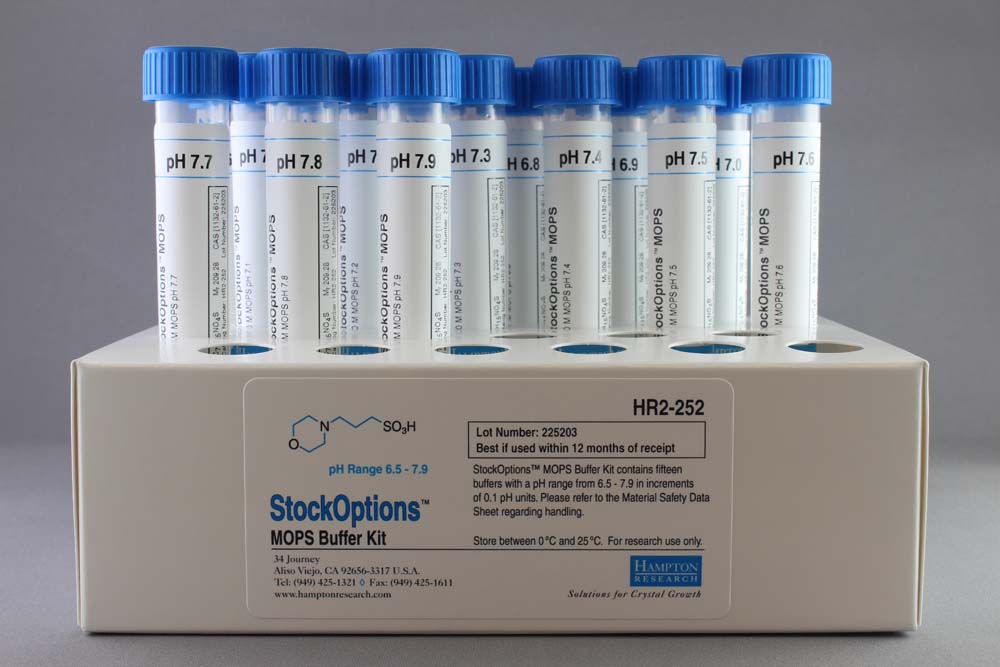
MOPS, Free Acid, Ultrol Grade - CAS 1132-61-2 - Calbiochem A zwitterionic buffer useful in the pH range of 6.5-7.9. Has a pKa of 7.20 at 25 C. Absorbance (1.0 M, H2O, 260 nm): 0.05. 1132-61-2

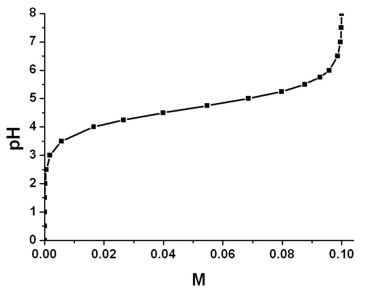
equilibrium - Why do buffers need to be composed of equal amounts of the acid and salt? - Chemistry Stack Exchange






![MOPS Running Buffer [10X] - Cepham Life Sciences Research Products MOPS Running Buffer [10X] - Cepham Life Sciences Research Products](https://www.cephamls.com/wp-content/uploads/2019/02/10379-MOPS_Running_Buffer_1-L-scaled.jpg)